
SL Paper 2
Titanium is a transition metal.
TiCl4 reacts with water and the resulting titanium(IV) oxide can be used as a smoke screen.
Describe the bonding in metals.
Titanium exists as several isotopes. The mass spectrum of a sample of titanium gave the following data:
Calculate the relative atomic mass of titanium to two decimal places.
State the number of protons, neutrons and electrons in the atom.
State the full electron configuration of the 2+ ion.
Explain why an aluminium-titanium alloy is harder than pure aluminium.
State the type of bonding in potassium chloride which melts at 1043 K.
A chloride of titanium, TiCl4, melts at 248 K. Suggest why the melting point is so much lower than that of KCl.
Formulate an equation for this reaction.
Suggest one disadvantage of using this smoke in an enclosed space.
Markscheme
electrostatic attraction
between «a lattice of» metal/positive ions/cations AND «a sea of» delocalized electrons
Accept mobile electrons.
Do not accept “metal atoms/nuclei”.
[2 marks]
= 47.93
Answer must have two decimal places with a value from 47.90 to 48.00.
Award [2] for correct final answer.
Award [0] for 47.87 (data booklet value).
[2 marks]
Protons: 22 AND Neutrons: 26 AND Electrons: 22
[1 mark]
1s22s22p63s23p63d2
[1 mark]
titanium atoms/ions distort the regular arrangement of atoms/ions
OR
titanium atoms/ions are a different size to aluminium «atoms/ions»
prevent layers sliding over each other
Accept diagram showing different sizes of atoms/ions.
[2 marks]
ionic
OR
«electrostatic» attraction between oppositely charged ions
[1 mark]
«simple» molecular structure
OR
weak«er» intermolecular bonds
OR
weak«er» bonds between molecules
Accept specific examples of weak bonds such as London/dispersion and van der Waals.
Do not accept “covalent”.
[1 mark]
TiCl4(l) + 2H2O(l) → TiO2(s) + 4HCl(aq)
correct products
correct balancing
Accept ionic equation.
Award M2 if products are HCl and a compound of Ti and O.
[2 marks]
HCl causes breathing/respiratory problems
OR
HCl is an irritant
OR
HCl is toxic
OR
HCl has acidic vapour
OR
HCl is corrosive
Accept “TiO2 causes breathing problems/is an irritant”.
Accept “harmful” for both HCl and TiO2.
Accept “smoke is asphyxiant”.
[1 mark]
Examiners report
The equations show steps in the formation and decomposition of ozone in the stratosphere, some of which absorb ultraviolet light.
Step 1 O2 → 2O•
Step 2 O• + O2 → O3
Step 3 O3 → O• + O2
Step 4 O• + O3 → 2O2
Draw the Lewis structures of oxygen, O2, and ozone, O3.
Outline why both bonds in the ozone molecule are the same length and predict the bond length in the ozone molecule. Refer to section 10 of the data booklet.
Reason:
Length:
Distinguish ultraviolet light from visible light in terms of wavelength and energy.
Discuss how the different bond strengths between the oxygen atoms in O2 and O3 in the ozone layer affect radiation reaching the Earth’s surface.
Markscheme
NOTES: Coordinate bond may be represented by an arrow.
Do not accept delocalized structure for ozone.
resonance «structures»
OR
delocalization of «the double/pi bond» electrons ✔
121 «pm» < length < 148 «pm» ✔
NOTE: Accept any length between these two values.
«UV» shorter wavelength AND higher energy «than visible» ✔
«bond» in O2 stronger than in O3 ✔
ozone absorbs lower frequency/energy «radiation than oxygen»
OR
ozone absorbs longer wavelength «radiation than oxygen» ✔
NOTE: Accept ozone «layer» absorbs a range of frequencies.
Examiners report
When heated in air, magnesium ribbon reacts with oxygen to form magnesium oxide.
The reaction in (a)(i) was carried out in a crucible with a lid and the following data was recorded:
Mass of crucible and lid = 47.372 ±0.001 g
Mass of crucible, lid and magnesium ribbon before heating = 53.726 ±0.001 g
Mass of crucible, lid and product after heating = 56.941 ±0.001 g
When magnesium is burnt in air, some of it reacts with nitrogen to form magnesium nitride according to the equation:
3 Mg (s) + N2 (g) → Mg3N2 (s)
The presence of magnesium nitride can be demonstrated by adding water to the product. It is hydrolysed to form magnesium hydroxide and ammonia.
Most nitride ions are 14N3–.
Write a balanced equation for the reaction that occurs.
State the block of the periodic table in which magnesium is located.
Identify a metal, in the same period as magnesium, that does not form a basic oxide.
Calculate the amount of magnesium, in mol, that was used.
Determine the percentage uncertainty of the mass of product after heating.
Assume the reaction in (a)(i) is the only one occurring and it goes to completion, but some product has been lost from the crucible. Deduce the percentage yield of magnesium oxide in the crucible.
Evaluate whether this, rather than the loss of product, could explain the yield found in (b)(iii).
Suggest an explanation, other than product being lost from the crucible or reacting with nitrogen, that could explain the yield found in (b)(iii).
Calculate coefficients that balance the equation for the following reaction.
__ Mg3N2 (s) + __ H2O (l) → __ Mg(OH)2 (s) + __ NH3 (aq)
Determine the oxidation state of nitrogen in Mg3N2 and in NH3.
Deduce, giving reasons, whether the reaction of magnesium nitride with water is an acid–base reaction, a redox reaction, neither or both.
State the number of subatomic particles in this ion.
Some nitride ions are 15N3–. State the term that describes the relationship between 14N3– and 15N3–.
The nitride ion and the magnesium ion are isoelectronic (they have the same electron configuration). Determine, giving a reason, which has the greater ionic radius.
Suggest two reasons why atoms are no longer regarded as the indivisible units of matter.
State the types of bonding in magnesium, oxygen and magnesium oxide, and how the valence electrons produce these types of bonding.
Markscheme
2 Mg(s) + O2(g) → 2 MgO(s) ✔
Do not accept equilibrium arrows. Ignore state symbols
s ✔
Do not allow group 2
aluminium/Al ✔
«mol» ✔
mass of product ✔
✔
Award [2] for correct final answer
Accept 0.021%
✔
✔
Award «0.2614 mol x 40.31 g mol–1»
Accept alternative methods to arrive at the correct answer.
Accept final answers in the range 91-92%
[2] for correct final answer.
yes
AND
«each Mg combines with N, so» mass increase would be 14x which is less than expected increase of 16x
OR
3 mol Mg would form 101g of Mg3N2 but would form 3 x MgO = 121 g of MgO
OR
0.2614 mol forms 10.536 g of MgO, but would form 8.796 g of Mg3N2 ✔
Accept Yes AND “the mass of N/N2 that combines with each g/mole of Mg is lower than that of O/O2”
Accept YES AND “molar mass of nitrogen less than of oxygen”.
incomplete reaction
OR
Mg was partially oxidised already
OR
impurity present that evaporated/did not react ✔
Accept “crucible weighed before fully cooled”.
Accept answers relating to a higher atomic mass impurity consuming less O/O2.
Accept “non-stoichiometric compounds formed”.
Do not accept "human error", "wrongly calibrated balance" or other non-chemical reasons.
If answer to (b)(iii) is >100%, accept appropriate reasons, such as product absorbed moisture before being weighed.
«1» Mg3N2 (s) + 6 H2O (l) → 3 Mg(OH)2 (s) + 2 NH3 (aq)
Mg3N2: -3
AND
NH3: -3 ✔
Do not accept 3 or 3-
Acid–base:
yes AND N3- accepts H+/donates electron pair«s»
OR
yes AND H2O loses H+ «to form OH-»/accepts electron pair«s» ✔
Redox:
no AND no oxidation states change ✔
Accept “yes AND proton transfer takes place”
Accept reference to the oxidation state of specific elements not changing.
Accept “not redox as no electrons gained/lost”.
Award [1 max] for Acid–base: yes AND Redox: no without correct reasons, if no other mark has been awarded
Protons: 7 AND Neutrons: 7 AND Electrons: 10 ✔
isotope«s» ✔
nitride AND smaller nuclear charge/number of protons/atomic number ✔
Any two of:
subatomic particles «discovered»
OR
particles smaller/with masses less than atoms «discovered»
OR
«existence of» isotopes «same number of protons, different number of neutrons» ✔
charged particles obtained from «neutral» atoms
OR
atoms can gain or lose electrons «and become charged» ✔
atom «discovered» to have structure ✔
fission
OR
atoms can be split ✔
Accept atoms can undergo fusion «to produce heavier atoms»
Accept specific examples of particles.
Award [2] for “atom shown to have a nucleus with electrons around it” as both M1 and M3.
Award [1] for all bonding types correct.
Award [1] for each correct description.
Apply ECF for M2 only once.
Examiners report
This was not as well done as one might have expected with the most common errors being O instead of O2 oxygen and MgO rather than MgO2.
Many students did not know what "block" meant, and often guessed group 2 etc.
Many students confused "period" and "group" and also many did not read metal, so aluminium was not chosen by the majority.
A number of students were not able to interpret the results and hence find the gain in mass and calculate the moles correctly.
Only a handful could work out the correct answer. Most had no real idea and quite a lot of blank responses. There also seems to be significant confusion between "percent uncertainty" and "percent error".
This was not well answered, but definitely better than the previous question with quite a few gaining some credit for correctly determining the theoretical yield.
This proved to be a very difficult question to answer in the quantitative manner required, with hardly any correct responses.
Quite a few students realised that incomplete reaction would lead to this, but only 30% of students gave a correct answer rather than a non-specific guess, such as "misread balance" or "impurities".
This was generally very well done with almost all candidates being able to determine the correct coefficients.
About 40% of students managed to correctly determine both the oxidation states, as -3, with errors being about equally divided between the two compounds.
Probably only about 10% could explain why this was an acid-base reaction. Rather more made valid deductions about redox, based on their answer to the previous question.
Most candidates could answer the question about subatomic particles correctly.
Identification of isotopes was answered correctly by most students.
In spite of being given the meaning of "isoelectronic", many candidates talked about the differing number of electrons and only about 30% could correctly analyse the situation in terms of nuclear charge.
The question was marked quite leniently so that the majority of candidates gained at least one of the marks by mentioning a subatomic particle. A significant number read "indivisible" as "invisible" however.
About a quarter of the students gained full marks and probably a similar number gained no marks. Metallic bonding was the type that seemed least easily recognised and least easily described. Another common error was to explain ionic bonding in terms of attraction of ions rather than describing electron transfer.
PCl5(g) and Cl2(g) were placed in a sealed flask and allowed to reach equilibrium at 200 °C. The enthalpy change, ΔH, for the decomposition of PCl5(g) is positive.
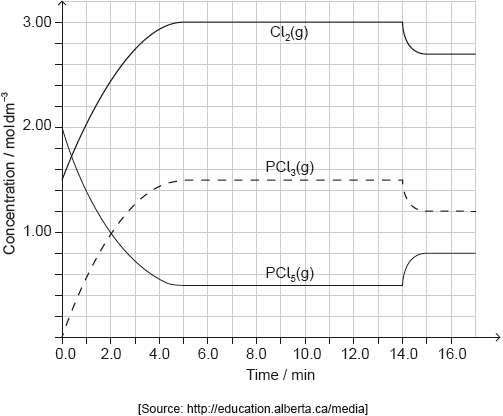
Deduce the equilibrium constant expression, Kc, for the decomposition of PCl5(g).
Deduce, giving a reason, the factor responsible for establishing the new equilibrium after 14 minutes.
Deduce the Lewis (electron dot) structure and molecular geometry of PCl3.
Markscheme
«Kc» =
[1 mark]
decrease in temperature
endothermic «reaction» AND «equilibrium» shifts to the left/reactants
OR
endothermic «reaction» AND Kc decreases
OR
endothermic «reaction» AND concentration of PCl5 increased/concentration of PCl3 and Cl2 decreased
OR
«equilibrium» shifts in exothermic direction
Do not accept “temperature change”.
Accept “ΔH positive” in place of “endothermic”.
Accept “products” instead of “PCl3 and Cl2”.
[2 marks]
Lewis structure:
Molecular geometry:
trigonal/triangular pyramidal
Penalize missing lone pairs once only between this question and 4(b).
Accept any combination of lines, dots or crosses to represent electrons.
Do not apply ECF.
[2 marks]
Examiners report
Butanoic acid, CH3CH2CH2COOH, is a weak acid and ethylamine, CH3CH2NH2, is a weak base.
State the equation for the reaction of each substance with water.
Explain why butanoic acid is a liquid at room temperature while ethylamine is a gas at room temperature.
State the formula of the salt formed when butanoic acid reacts with ethylamine.
Markscheme
Butanoic acid:
CH3CH2CH2COOH (aq) + H2O (l) CH3CH2CH2COO− (aq) + H3O+ (aq) ✔
Ethylamine:
CH3CH2NH2 (aq) + H2O (l) CH3CH2NH3+ (aq) + OH− (aq) ✔
Any two of:
butanoic acid forms more/stronger hydrogen bonds ✔
butanoic acid forms stronger London/dispersion forces ✔
butanoic acid forms stronger dipole–dipole interaction/force ✔
Accept “butanoic acid forms dimers”
Accept “butanoic acid has larger Mr/hydrocarbon chain/number of electrons” for M2.
Accept “butanoic acid has larger «permanent» dipole/more polar” for M3.
CH3CH2NH3+ CH3CH2CH2COO−
OR
CH3CH2CH2COO− CH3CH2NH3+
OR
CH3CH2CH2COO− H3N+CH2CH3 ✔
The charges are not necessary for the mark.
Examiners report
Magnetite, Fe3O4, is another ore of iron that contains both Fe2+ and Fe3+.
Iron exists as several isotopes.
In acidic solution, hydrogen peroxide, H2O2, will oxidize Fe2+.
Fe2+ (aq) → Fe3+ (aq) + e−
Deduce the ratio of Fe2+:Fe3+ in Fe3O4.
State the type of spectroscopy that could be used to determine their relative abundances.
State the number of protons, neutrons and electrons in each species.
Iron has a relatively small specific heat capacity; the temperature of a 50 g sample rises by 44.4°C when it absorbs 1 kJ of heat energy.
Determine the specific heat capacity of iron, in J g−1 K−1. Use section 1 of the data booklet.
Write the half-equation for the reduction of hydrogen peroxide to water in acidic solution.
Deduce a balanced equation for the oxidation of Fe2+ by acidified hydrogen peroxide.
Markscheme
1:2 ✔
Accept 2 Fe3+: 1 Fe2+
Do not accept 2:1 only
mass «spectroscopy»/MS ✔
Award [1 max] for 4 correct values.
specific heat capacity « = » = 0.45 «J g−1 K−1» ✔
H2O2(aq) + 2H+(aq) + 2e−→ 2H2O(l) ✔
H2O2(aq) + 2H+(aq) + 2Fe2+(aq) → 2H2O(l) + 2Fe3+(aq) ✔
Examiners report
Bonds can be formed in many ways.
The landing module for the Apollo mission used rocket fuel made from a mixture of hydrazine, N2H4, and dinitrogen tetraoxide, N2O4.
N2H4(l) + N2O4(l) → 3N2(g) + 4H2O(g)
State and explain the difference in bond strength between the nitrogen atoms in a hydrazine and nitrogen molecule.
State why hydrazine has a higher boiling point than dinitrogen tetraoxide.
Determine the oxidation state of nitrogen in the two reactants.
Deduce, giving a reason, which species is the reducing agent.
Deduce the Lewis (electron dot) structures of ozone.
Markscheme
triple bond in nitrogen «molecule» AND single bond in hydrazine
triple bond stronger than single bond
OR
more shared «pairs of» electrons make bond stronger/attract nuclei more
Accept bond enthalpy values from data booklet (158 and 945 kJmol–1).
[2 marks]
hydrogen bonding «between molecules, dinitrogen tetraoxide does not»
[1 mark]
N2H4: –2 AND N2O4: +4
[1 mark]
N2H4 AND oxidized/oxidation state increases
OR
N2H4 AND loses hydrogen
OR
N2H4 AND reduces/removes oxygen from N2O4
Accept “N2H4 AND gives electrons «to N2O4»”.
[1 mark]
Accept any combination of lines, dots or crosses to represent electrons.
Do not penalize missing lone pairs if already done in 3b.
Do not accept structure that represents 1.5 bonds.
[2 marks]
Examiners report
White phosphorus is an allotrope of phosphorus and exists as P4.
An equilibrium exists between PCl3 and PCl5.
PCl3 (g) + Cl2 (g) PCl5 (g)
Sketch the Lewis (electron dot) structure of the P4 molecule, containing only single bonds.
Write an equation for the reaction of white phosphorus (P4) with chlorine gas to form phosphorus trichloride (PCl3).
Deduce the electron domain and molecular geometry using VSEPR theory, and estimate the Cl–P–Cl bond angle in PCl3.
Explain the polarity of PCl3.
Calculate the standard enthalpy change (ΔH⦵) for the forward reaction in kJ mol−1.
ΔH⦵f PCl3 (g) = −306.4 kJ mol−1
ΔH⦵f PCl5 (g) = −398.9 kJ mol−1
State the equilibrium constant expression, Kc, for this reaction.
State, with a reason, the effect of an increase in temperature on the position of this equilibrium.
Markscheme
Accept any diagram with each P joined to the other three.
Accept any combination of dots, crosses and lines.
P4 (s) + 6Cl2 (g) → 4PCl3 (l) ✔
Electron domain geometry: tetrahedral ✔
Molecular geometry: trigonal pyramidal ✔
Bond angle: 100«°» ✔
Accept any value or range within the range 91−108«°» for M3.
polar AND unsymmetrical distribution of charge
OR
polar AND dipoles do not cancel
OR
«polar as» dipoles «add to» give a «partial» positive «charge» at P and a «partial» negative «charge» at the opposite/Cl side of the molecule ✔
Accept “polar AND unsymmetrical molecule”.
«−398.9 kJ mol−1 − (−306.4 kJ mol−1) =» −92.5 «kJ mol−1» ✔
«Kc =» ✔
«shifts» left/towards reactants AND «forward reaction is» exothermic/ΔH is negative ✔
Examiners report
Ethane-1,2-diol, HOCH2CH2OH, has a wide variety of uses including the removal of ice from aircraft and heat transfer in a solar cell.
Ethane-1,2-diol can be formed according to the following reaction.
2CO (g) + 3H2 (g) HOCH2CH2OH (g)
(i) Deduce the equilibrium constant expression, Kc, for this reaction.
(ii) State how increasing the pressure of the reaction mixture at constant temperature will affect the position of equilibrium and the value of Kc.
Position of equilibrium:
Kc:
(iii) Calculate the enthalpy change, ΔHθ, in kJ, for this reaction using section 11 of the data booklet. The bond enthalpy of the carbon–oxygen bond in CO (g) is 1077kJmol-1.
(iv) The enthalpy change, ΔHθ, for the following similar reaction is –233.8 kJ.
2CO(g) + 3H2(g) HOCH2CH2OH (l)
Deduce why this value differs from your answer to (a)(iii).
Determine the average oxidation state of carbon in ethene and in ethane-1,2-diol.
Ethene:
Ethane-1,2-diol:
Explain why the boiling point of ethane-1,2-diol is significantly greater than that of ethene.
Ethane-1,2-diol can be oxidized first to ethanedioic acid, (COOH)2, and then to carbon dioxide and water. Suggest the reagents to oxidize ethane-1,2-diol.
Markscheme
(i)
(ii)
Position of equilibrium: moves to right OR favours product
Kc: no change OR is a constant at constant temperature
(iii)
Bonds broken: 2C≡O + 3(H-H) / 2(1077kJmol-1) + 3(436kJmol-1) / 3462 «kJ»
Bonds formed: 2(C-O) + 2(O-H) + 4(C-H) + (C-C) / 2(358kJmol-1) + 2(463kJmol-1) + 4(414kJmol-1) + 346kJmol-1 / 3644 «kJ»
«Enthalpy change = bonds broken - bonds formed = 3462 kJ - 3644 kJ =» -182 «kJ»
Award [3] for correct final answer.
Award [2 max] for «+»182 «kJ».
(iv)
in (a)(iii) gas is formed and in (a)(iv) liquid is formed
OR
products are in different states
OR
conversion of gas to liquid is exothermic
OR
conversion of liquid to gas is endothermic
OR
enthalpy of vapourisation needs to be taken into account
Accept product is «now» a liquid.
Accept answers referring to bond enthalpies being means/averages.
Ethene: –2
Ethane-1,2-diol: –1
Do not accept 2–, 1– respectively.
ethane-1,2-diol can hydrogen bond to other molecules «and ethene cannot»
OR
ethane-1,2-diol has «significantly» greater van der Waals forces
Accept converse arguments.
Award [0] if answer implies covalent bonds are broken
hydrogen bonding is «significantly» stronger than other intermolecular forces
acidified «potassium» dichromate«(VI)»/H+ AND K2Cr2O7/H+ AND Cr2O72-
OR
«acidified potassium» manganate(VII)/ «H+» KMnO4 /«H+» MnO4-
Accept Accept H2SO4 or H3PO4 for H+.
Accept “permanganate” for “manganate(VII)”.
Examiners report
This question is about compounds of sodium.
Sodium peroxide is used in diving apparatus to produce oxygen from carbon dioxide.
2Na2O2 (s) + 2CO2 (g) → 2Na2CO3 (s) + O2 (g)
Describe the structure and bonding in solid sodium oxide.
Write equations for the separate reactions of solid sodium oxide and solid phosphorus(V) oxide with excess water and differentiate between the solutions formed.
Sodium oxide, Na2O:
Phosphorus(V) oxide, P4O10:
Differentiation:
Sodium peroxide, Na2O2, is formed by the reaction of sodium oxide with oxygen.
2Na2O (s) + O2 (g) → 2Na2O2 (s)
Calculate the percentage yield of sodium peroxide if 5.00 g of sodium oxide produces 5.50 g of sodium peroxide.
Determine the enthalpy change, ΔH, in kJ, for this reaction using data from the table and section 12 of the data booklet.
Outline why bond enthalpy values are not valid in calculations such as that in (c)(i).
The reaction of sodium peroxide with excess water produces hydrogen peroxide and one other sodium compound. Suggest the formula of this compound.
State the oxidation number of carbon in sodium carbonate, Na2CO3.
Markscheme
«3-D/giant» regularly repeating arrangement «of ions»
OR
lattice «of ions» [✔]
electrostatic attraction between oppositely charged ions
OR
electrostatic attraction between Na+ and O2− ions [✔]
Note: Do not accept “ionic” without description.
Sodium oxide:
Na2O(s) + H2O(l) → 2NaOH (aq) [✔]
Phosphorus(V) oxide:
P4O10 (s) + 6H2O(l) → 4H3PO4 (aq) [✔]
Differentiation:
NaOH / product of Na2O is alkaline/basic/pH > 7 AND H3PO4 / product of P4O10 is acidic/pH < 7 [✔]
n(Na2O2) theoretical yield «= » = 0.0807/8.07 × 10−2 «mol»
OR
mass Na2O2 theoretical yield «= × 77.98 gmol−1» = 6.291 «g» [✔]
% yield «= × 100» OR « x 100» = 87.4 «%» [✔]
Note: Award [2] for correct final answer.
ΣΔHf products = 2 × (−1130.7) / −2261.4 «kJ» [✔]
ΣΔHf reactants = 2 × (−510.9) + 2 × (−393.5) / −1808.8 «kJ» [✔]
ΔH = «ΣΔHf products − ΣΔHf reactants = −2261.4 −(−1808.8) =» −452.6 «kJ» [✔]
Note: Award [3] for correct final answer.
Award [2 max] for “+452.6 «kJ»”.
only valid for covalent bonds
OR
only valid in gaseous state [✔]
NaOH [✔]
Note: Accept correct equation showing NaOH as a product.
IV [✔]
Examiners report
Disappointingly many students did not realise that sodium oxide was held by ionic bonds, many said it was covalent or metallic bonding. The ones that knew it was ionic failed to describe it adequately to earn the 2 marks.
Very few students could correctly write out the two equations and so often were unable to realise it was acid/base behaviour that would differentiate the oxides.
Many candidates were able to correctly calculate the % yield but some weaker candidates just used 5.0/5.5 to find %.
The calculation of the enthalpy change using enthalpies of formation was generally answered well but common mistakes were students forgetting to multiply by 2 or adding extra terms for oxygen.
Most students didn’t gain a mark and “values are average” was the most common incorrect answer. The fact this was an ionic compound did not register with them. Some students did gain a mark for stating that the substances were not in a gaseous state.
Some students correctly identified sodium hydroxide as the correct product, but hydrogen, oxygen and sodium oxide were common answers.
Oxidation number of +4 was often correctly identified.
Ethyne, C2H2, reacts with oxygen in welding torches.
Ethyne reacts with steam.
C2H2 (g) + H2O (g) → C2H4O (g)
Two possible products are:
Product B, CH3CHO, can also be synthesized from ethanol.
Write an equation for the complete combustion of ethyne.
Deduce the Lewis (electron dot) structure of ethyne.
Compare, giving a reason, the length of the bond between the carbon atoms in ethyne with that in ethane, C2H6.
Identify the type of interaction that must be overcome when liquid ethyne vaporizes.
Product A contains a carbon–carbon double bond. State the type of reactions that compounds containing this bond are likely to undergo.
State the name of product B, applying IUPAC rules.
Determine the enthalpy change for the reaction, in kJ, to produce A using section 11 of the data booklet.
The enthalpy change for the reaction to produce B is −213 kJ. Predict, giving a reason, which product is the most stable.
The IR spectrum and low resolution 1H NMR spectrum of the actual product formed are shown.
Deduce whether the product is A or B, using evidence from these spectra together with sections 26 and 27 of the data booklet.
Identity of product:
One piece of evidence from IR:
One piece of evidence from 1H NMR:
Suggest the reagents and conditions required to ensure a good yield of product B.
Reagents:
Conditions:
Deduce the average oxidation state of carbon in product B.
Explain why product B is water soluble.
Markscheme
C2H2 (g) + 2.5O2 (g) → 2CO2 (g) + H2O (l)
OR
2C2H2 (g) + 5O2 (g) → 4CO2 (g) + 2H2O (l) [✔]
[✔]
Note: Accept any valid combination of lines, dots and crosses.
«ethyne» shorter AND a greater number of shared/bonding electrons
OR
«ethyne» shorter AND stronger bond [✔]
London/dispersion/instantaneous dipole-induced dipole forces [✔]
Note: Do not accept just “intermolecular forces” or “van der Waals’ forces”.
«electrophilic» addition/A«E» [✔]
Note: Accept “polymerization”.
ethanal [✔]
«sum of bond enthalpies of reactants =» 2(C–H) + C≡C + 2(O–H)
OR
2 × 414 «kJ mol–1» + 839 «kJ mol–1» + 2 × 463 «kJ mol–1»
OR
2593 «kJ» [✔]
«sum of bond enthalpies of A =» 3(C–H) + C=C + C–O + O–H
OR
3 × 414 «kJ mol–1» + 614 «kJ mol–1» + 358 «kJ mol–1» + 463 «kJ mol–1»
OR
2677 «kJ» [✔]
«enthalpy of reaction = 2593 kJ – 2677 kJ» = –84 «kJ» [✔]
Note: Award [3] for correct final answer.
B AND it has a more negative/lower enthalpy/«potential» energy
OR
B AND more exothermic «enthalpy of reaction from same starting point» [✔]
Identity of product: «B»
IR spectrum:
1700–1750 «cm–1 band» AND carbonyl/CO group present
OR
no «band at» 1620–1680 «cm–1» AND absence of double bond/C=C
OR
no «broad band at» 3200–3600 «cm–1» AND absence of hydroxyl/OH group [✔]
Note: Accept a specific value or range of wavenumbers and chemical shifts.
1H NMR spectrum:
«only» two signals AND A would have three
OR
«signal at» 9.4–10.0 «ppm» AND «H atom/proton of» aldehyde/–CHO present
OR
«signal at» 2.2–2.7 «ppm» AND «H atom/proton of alkyl/CH next to» aldehyde/CHO present
OR
«signal at» 2.2–2.7 «ppm» AND «H atom/proton of» RCOCH2- present
OR
no «signal at» 4.5–6.0 «ppm» AND absence of «H-atom/proton next to» double bond/C=C [✔]
Note: Accept “two signals with areas 1:3”.
Reagents:
acidified/H+ AND «potassium» dichromate«(VI)»/K2Cr2O7/Cr2O72– [✔]
Conditions:
distil «the product before further oxidation» [✔]
Note: Accept “«acidified potassium» manganate(VII)/KMnO4/MnO4–/permanganate”.
Accept “H2SO4” or “H3PO4” for “H+”.
Accept “more dilute dichromate(VI)/manganate(VII)” or “excess ethanol”.
Award M1 if correct reagents given under “Conditions”.
–1 [✔]
Any three of:
has an oxygen/O atom with a lone pair [✔]
that can form hydrogen bonds/H-bonds «with water molecules» [✔]
hydrocarbon chain is short «so does not disrupt many H-bonds with water molecules» [✔]
«large permanent» dipole-dipole interactions with water [✔]
Examiners report
Almost all candidates recognized the products of the complete combustion of ethyne, and over two thirds managed to balance the equation. It was good to see candidates using integers for the balancing.
The majority of candidates drew the Lewis structure of ethyne. A few teachers commented that they did not cover alkynes assuming they are not included in the syllabus. Please check the current syllabus carefully when preparing students.
A very well answered question. The vast majority of candidates understood that triple bonds are stronger than single bonds and result in a shorter bond length. It was disappointing, however, to see a considerable number of candidates stating that ethane has a double bond.
Some candidates could not relate evaporation of a liquid to the breaking of its intermolecular forces and gave irrelevant answers such as “evaporation”. Other candidates gave general answers such as “the intermolecular forces” or used the term “van der Waals’ forces” which did not gain credit as too vague. The current guide is clear that “London/dispersion forces” is the appropriate term to use for instantaneous dipole-induced dipole forces. Less than 40 % of the candidates scored the mark. It was disappointing to see some candidates state “covalent bonding” as the type of interaction that must be overcome when liquid ethyne vaporizes. Some teachers thought the wording of the question may have been vague and candidates may have been confused about what was meant by the “type of interaction”.
About 60 % of the candidates stated “addition” as the type of reactions that compounds containing carbon-carbon double bonds underwent. It was disappointing to see a variety of answers including substitution, condensation and combustion showing a total lack of understanding. Some candidates gave specific types such as "bromination" or “hydration” which did not receive the mark.
60 % of the candidates were able to name compound B as ethanal. Some candidates did not recognize it as an aldehyde and gave names related to carboxylic acids or other homologous series. Other candidates called it methanal.
Candidates were confident in using average bond enthalpies for calculating the enthalpy change for the reaction. Mistakes included forgetting to include the breaking of the O-H bonds in water and reversing the signs.
Reasonably well answered. About half of the candidates showed understanding of the relation between stability and the enthalpy change from the same starting materials. ECF was applied in this question based on the answer in part (iii).
The majority of candidates handled this question competently and nearly half of the candidates obtained both marks. They obtained the value of the absorption from the spectra provided and compared it to the values in the data booklet to deduce the identity of the product. Common mistakes included not identifying the peaks and signals precisely (for example C=O instead of CHO for 1H NMR signal at 9.4-10.0 ppm). Some teachers commented that the TMS signal should not have been included as the SL do not know about it. Other teachers commented that using the 'actual' rather than an ‘idealized’ IR spectrum may have caused confusion due to the peak at around 3400 cm-1 which could be confused for O-H in alcohols. Thankfully both of these answers were hardly seen in the scripts. The peak at 3400 cm-1 was not at all broad and did not confuse the majority of students. Please note that real spectra are usually used in examination papers, and it is worth encouraging students to check more than one peak to confirm their deductions.
Surprisingly, this question was not answered well by the majority of the candidates. However, it did discriminate well between high-scoring and low-scoring candidates. Common mistakes included incorrect formulas (such as K2CrO7), missing the acidic conditions and stating “reflux” instead of “distillation”. Many candidates gave completely irrelevant reagents and conditions such as “oxygen, pressure and a nickel catalyst”. It is possible that some candidates did not think of “distillation” as a “condition”.
About 60 % of the candidates determined the average oxidation state of carbon in ethanal. A couple of teachers commented that asking SL students to determine an “average oxidation state” seems a little difficult. Please note that this term has been used in recent papers whenever there are two or more atoms of the element in different parts of the compound. There was no evidence of confusion on the part of the candidates and most answered the question well.
This was a challenging question with a demanding markscheme. Most students missed the fact that ethanal can form hydrogen bonds with water. And students who did state this often achieved only 1 out of the 3 marks because they did not offer a full explanation. Some candidates stating "hydrogen bonding" showed confusion by mentioning the hydrogen of the aldehyde group. Few identified the lone pairs on oxygen as the reason for the ability to hydrogen bond. Most candidates just stated that ethanal is polar and dissolves in polar water achieving no marks. However, one mark was awarded for “dipole-dipole interactions with water”.
Sulfur trioxide is produced from sulfur dioxide.
2SO2 (g) + O2 (g) 2SO3 (g) ΔH = −196 kJ mol−1
The reaction between sulfur dioxide and oxygen can be carried out at different temperatures.
Nitric acid, HNO3, is another strong Brønsted–Lowry acid. Its conjugate base is the nitrate ion, NO3−
Outline, giving a reason, the effect of a catalyst on a reaction.
On the axes, sketch Maxwell–Boltzmann energy distribution curves for the reacting species at two temperatures T1 and T2, where T2 > T1.
Explain the effect of increasing temperature on the yield of SO3.
State the product formed from the reaction of SO3 with water.
State the meaning of a strong Brønsted–Lowry acid.
Draw the Lewis structure of NO3−.
Explain the electron domain geometry of NO3−.
Markscheme
increases rate AND lower Ea ✔
provides alternative pathway «with lower Ea»
OR
more/larger fraction of molecules have the «lower» Ea ✔
Accept description of how catalyst lowers Ea for M2 (e.g. “reactants adsorb on surface «of catalyst»”, “reactant bonds weaken «when adsorbed»”, “helps favorable orientation of molecules”).
both axes correctly labelled ✔
peak of T2 curve lower AND to the right of T1 curve ✔
lines begin at origin AND correct shape of curves AND T2 must finish above T1 ✔
Accept “probability «density» / number of particles / N / fraction” on y-axis.
Accept “kinetic E/KE/Ek” but not just “Energy/E” on x-axis.
decrease AND equilibrium shifts left / favours reverse reaction ✔
«forward reaction is» exothermic / ΔH is negative ✔
sulfuric acid/H2SO4 ✔
Accept “disulfuric acid/H2S2O7”.
fully ionizes/dissociates ✔
proton/H+ «donor » ✔
Do not accept the delocalised structure.
Accept any combination of dots, crosses and lines.
Coordinate/dative bond may be represented by an arrow.
three electron domains repel
OR
three electron domains as far away as possible ✔
trigonal planar
OR
«all» angles are 120° ✔
Examiners report
A generally well-answered question. Most candidates explained the effect of a catalyst on a reaction correctly. A small proportion of candidates thought the catalyst increased the frequency of collisions. Some candidates focussed on the effect of the catalyst on an equilibrium since the equation above the question was that of a reversible reaction. These candidates usually still managed to gain at least the first marking point by stating that both forward and reverse reaction rates were increased due to the lower activation energy. Most candidates mentioned the alternative pathway for the second mark, and some gave a good discussion about the increase in the number of molecules or collisions with E≥Ea. A few candidates lost one of the marks for not explicitly stating the effect of a catalyst (that it increases the rate of the reaction).
The average mark scored for the Maxwell-Boltzmann distribution curves sketch was 1.5 out of 3 marks and the question had a strong correlation with the candidates who did well overall. The majority of candidates were familiar with the shapes of the curves. The most commonly lost mark was missing or incorrect labels on the axes. Sometimes candidates added the labels but did not specify “kinetic” energy for the x-axis. As for the curves, some candidates reversed the labels T1 and T2, some made the two curves meet at high energy or even cross, and some did not have the correct relationship between the peaks of T1 and T2.
Another question that showed a strong correlation with the candidates who did well overall. The average mark was 1 out of 2 marks. Many candidates explained the effect of an increase in temperature on the yield of SO3 correctly and thoroughly. One of the common mistakes was to miss the fact that it was an equilibrium and reason that yield would not change due to an increase in the rate of reaction. Unfortunately, a number of candidates also deduced that yield would increase due to the increase in rate. Other candidates recognized that it was an exothermic reaction but deduced the equilibrium would shift to the right giving a higher yield of SO3.
A very well answered question. 70% of the candidates stated H2SO4 as the product from the reaction of SO3 with water.
While a straightforward question, many candidates only answered part of the question - either focussing on the “strong” or on the “Brønsted-Lowry acid”. The average mark on this question was 1.2 out of 2 marks.
Only 20% of the candidates scored the mark for the Lewis structure of NO3-. Mistakes included: missing charge, missing lone pairs, 3 single bonds, 2 double bonds.
The majority of candidates deduced the correct electron domain geometry scoring the first mark including cases of ECF. Only a small number of candidates satisfied the requirements of the markscheme for the explanation.
Automobile air bags inflate by a rapid decomposition reaction. One typical compound used is guanidinium nitrate, C(NH2)3NO3, which decomposes very rapidly to form nitrogen, water vapour and carbon.
Deduce the equation for the decomposition of guanidinium nitrate.
Calculate the total number of moles of gas produced from the decomposition of 10.0 g of guanidinium nitrate.
Calculate the pressure, in kPa, of this gas in a 10.0 dm3 air bag at 127°C, assuming no gas escapes.
Suggest why water vapour deviates significantly from ideal behaviour when the gases are cooled, while nitrogen does not.
Another airbag reactant produces nitrogen gas and sodium.
Suggest, including an equation, why the products of this reactant present a safety hazard.
Markscheme
C(NH2)3NO3 (s) → 2N2 (g) + 3H2O (g) + C (s) ✔
moles of gas = « » 0.409 «mol» ✔
«» = 136 «kPa» ✔
Any two of:
nitrogen non-polar/London/dispersion forces AND water polar/H-bonding ✔
water has «much» stronger intermolecular forces ✔
water molecules attract/condense/occupy smaller volume «and therefore deviate from ideal behaviour» ✔
2Na (s) + 2H2O (l) → 2NaOH (aq) + H2 (g) ✔
hydrogen explosive
OR
highly exothermic reaction
OR
sodium reacts violently with water
OR
forms strong alkali ✔
NOTE: Accept the equation of combustion of hydrogen.
Do not accept just “sodium is reactive/dangerous”.
Examiners report
Calcium carbide, CaC2, is an ionic solid.
Describe the nature of ionic bonding.
State the electron configuration of the Ca2+ ion.
When calcium compounds are introduced into a gas flame a red colour is seen; sodium compounds give a yellow flame. Outline the source of the colours and why they are different.
Suggest two reasons why solid calcium has a greater density than solid potassium.
Outline why solid calcium is a good conductor of electricity.
Calcium carbide reacts with water to form ethyne and calcium hydroxide.
CaC2(s) + H2O(l) → C2H2(g) + Ca(OH)2(aq)
Estimate the pH of the resultant solution.
Markscheme
electrostatic attraction AND oppositely charged ions
[1 mark]
1s22s22p63s23p6
OR
[Ar]
[1 mark]
«promoted» electrons fall back to lower energy level
energy difference between levels is different
Accept “Na and Ca have different nuclear charge” for M2.
[2 marks]
Any two of:
stronger metallic bonding
smaller ionic/atomic radius
two electrons per atom are delocalized
OR
greater ionic charge
greater atomic mass
Do not accept just “heavier” or “more massive” without reference to atomic mass.
[2 marks]
delocalized/mobile electrons «free to move»
[1 mark]
pH > 7
Accept any specific pH value or range of values above 7 and below 14.
[1 mark]
Examiners report
Urea, (H2N)2CO, is excreted by mammals and can be used as a fertilizer.
Calculate the percentage by mass of nitrogen in urea to two decimal places using section 6 of the data booklet.
Suggest how the percentage of nitrogen affects the cost of transport of fertilizers giving a reason.
The structural formula of urea is shown.
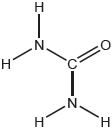
Predict the electron domain and molecular geometries at the nitrogen and carbon atoms, applying the VSEPR theory.
Urea can be made by reacting potassium cyanate, KNCO, with ammonium chloride, NH4Cl.
KNCO(aq) + NH4Cl(aq) → (H2N)2CO(aq) + KCl(aq)
Determine the maximum mass of urea that could be formed from 50.0 cm3 of 0.100 mol dm−3 potassium cyanate solution.
Urea can also be made by the direct combination of ammonia and carbon dioxide gases.
2NH3(g) + CO2(g) (H2N)2CO(g) + H2O(g) ΔH < 0
Predict, with a reason, the effect on the equilibrium constant, Kc, when the temperature is increased.
Suggest one reason why urea is a solid and ammonia a gas at room temperature.
Sketch two different hydrogen bonding interactions between ammonia and water.
The combustion of urea produces water, carbon dioxide and nitrogen.
Formulate a balanced equation for the reaction.
The mass spectrum of urea is shown below.
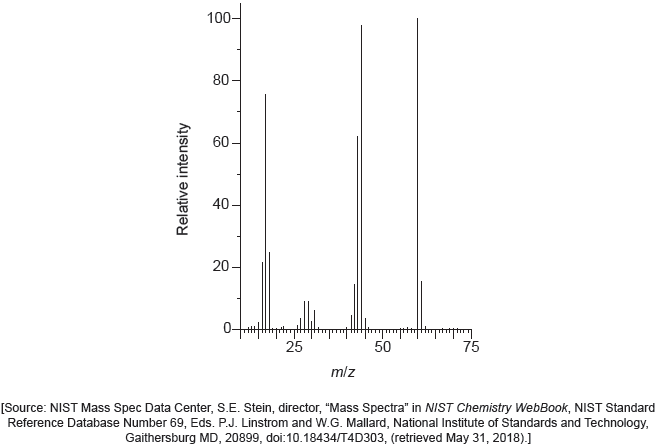
Identify the species responsible for the peaks at m/z = 60 and 44.
The IR spectrum of urea is shown below.
Identify the bonds causing the absorptions at 3450 cm−1 and 1700 cm−1 using section 26 of the data booklet.
Predict the number of signals in the 1H NMR spectrum of urea.
Markscheme
molar mass of urea «= 4 × 1.01 + 2 × 14.01 + 12.01 + 16.00» = 60.07 «g mol–1»
«% nitrogen = × 100 =» 46.65 «%»
Award [2] for correct final answer.
Award [1 max] for final answer not to two decimal places.
[2 marks]
«cost» increases AND lower N% «means higher cost of transportation per unit of nitrogen»
OR
«cost» increases AND inefficient/too much/about half mass not nitrogen
Accept other reasonable explanations.
Do not accept answers referring to safety/explosions.
[1 mark]
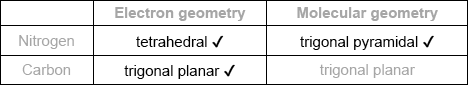
Note: Urea’s structure is more complex than that predicted from VSEPR theory.
[3 marks]
n(KNCO) «= 0.0500 dm3 × 0.100 mol dm–3» = 5.00 × 10–3 «mol»
«mass of urea = 5.00 × 10–3 mol × 60.07 g mol–1» = 0.300 «g»
Award [2] for correct final answer.
[2 marks]
«Kc» decreases AND reaction is exothermic
OR
«Kc» decreases AND ΔH is negative
OR
«Kc» decreases AND reverse/endothermic reaction is favoured
[1 mark]
Any one of:
urea has greater molar mass
urea has greater electron density/greater London/dispersion
urea has more hydrogen bonding
urea is more polar/has greater dipole moment
Accept “urea has larger size/greater van der Waals forces”.
Do not accept “urea has greater intermolecular forces/IMF”.
[1 mark]
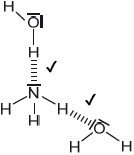
Award [1] for each correct interaction.
If lone pairs are shown on N or O, then the lone pair on N or one of the lone pairs on O MUST be involved in the H-bond.
Penalize solid line to represent H-bonding only once.
[2 marks]
2(H2N)2CO(s) + 3O2(g) → 4H2O(l) + 2CO2(g) + 2N2(g)
correct coefficients on LHS
correct coefficients on RHS
Accept (H2N)2CO(s) + O2(g) → 2H2O(l) + CO2(g) + N2(g).
Accept any correct ratio.
[2 marks]
60: CON2H4+
44: CONH2+
Accept “molecular ion”.
[2 marks]
3450 cm–1: N–H
1700 cm–1: C=O
Do not accept “O–H” for 3450 cm–1.
[2 marks]
1
[1 mark]
Examiners report
Benzoic acid, C6H5COOH, is another derivative of benzene.
Draw the structure of the conjugate base of benzoic acid showing all the atoms and all the bonds.
The pH of an aqueous solution of benzoic acid at 298 K is 2.95. Determine the concentration of hydroxide ions in the solution, using section 2 of the data booklet.
Formulate the equation for the complete combustion of benzoic acid in oxygen using only integer coefficients.
Suggest how benzoic acid, Mr = 122.13, forms an apparent dimer, Mr = 244.26, when dissolved in a non-polar solvent such as hexane.
Markscheme
[✔]
Note: Accept Kekulé structures.
Negative sign must be shown in correct position- on the O or delocalised over the carboxylate.
ALTERNATIVE 1:
[H+] «= 10−2.95» = 1.122 × 10−3 «mol dm−3» [✔]
«[OH−] = =» 8.91 × 10−12 «mol dm−3» [✔]
ALTERNATIVE 2:
pOH = «14 − 2.95 =» 11.05 [✔]
«[OH−] = 10−11.05 =» 8.91 × 10−12 «moldm−3» [✔]
Note: Award [2] for correct final answer.
Accept other methods.
2C6H5COOH(s) + 15O2 (g) → 14CO2 (g) + 6H2O(l)
correct products [✔]
correct balancing [✔]
«intermolecular» hydrogen bonding [✔]
Note: Accept diagram showing hydrogen bonding.
Examiners report
Most failed to score a mark for the conjugate base of benzoic acid as either they didn’t show all bonds and atoms in the ring and/or they did not put the minus sign in the correct place. Some didn't read the question carefully so gave the structure of the acid form.
Many students could correctly calculate the hydroxide concentration, but some weaker students calculated hydrogen ion concentration only.
Most students earned at least one mark for writing the correct products of the combustion of benzoic acid but the balancing appeared to be difficult for some.
Very few students answered this question correctly, thinking benzoic would bond with the hexane even though it was a non-polar solvent. It was very rare for a student to realize there was intermolecular hydrogen bonding.
The concentration of a solution of a weak acid, such as ethanedioic acid, can be determined
by titration with a standard solution of sodium hydroxide, NaOH (aq).
Distinguish between a weak acid and a strong acid.
Weak acid:
Strong acid:
Suggest why it is more convenient to express acidity using the pH scale instead of using the concentration of hydrogen ions.
5.00 g of an impure sample of hydrated ethanedioic acid, (COOH)2•2H2O, was dissolved in water to make 1.00 dm3 of solution. 25.0 cm3 samples of this solution were titrated against a 0.100 mol dm-3 solution of sodium hydroxide using a suitable indicator.
(COOH)2 (aq) + 2NaOH (aq) → (COONa)2 (aq) + 2H2O (l)
The mean value of the titre was 14.0 cm3.
(i) Calculate the amount, in mol, of NaOH in 14.0 cm3 of 0.100 mol dm-3 solution.
(ii) Calculate the amount, in mol, of ethanedioic acid in each 25.0 cm3 sample.
(iii) Determine the percentage purity of the hydrated ethanedioic acid sample.
The Lewis (electron dot) structure of the ethanedioate ion is shown below.
Outline why all the C–O bond lengths in the ethanedioate ion are the same length and suggest a value for them. Use section 10 of the data booklet.
Markscheme
Weak acid: partially dissociated/ionized «in solution/water»
AND
Strong acid: «assumed to be almost» completely/100% dissociated/ionized «in solution/water»
Accept answers relating to pH, conductivity, reactivity if solutions of equal concentrations stated.
«log scale» reduces a wide range of numbers to a small range
OR
simple/easy to use
OR
converts exponential expressions into linear scale/simple numbers
Do not accept “easy for calculations”
i
«n(NaOH) = dm-3 x 0.100 mol dm-3 =» 1.40 x 10-3 «mol»
ii
« «mol»
iii
ALTERNATIVE 1:
«mass of pure hydrated ethanedioic acid in each titration = 7.00 × 10-4 mol × 126.08 g mol-1 =» 0.0883 / 8.83 × 10-2 «g»
mass of sample in each titration = «×5.00g=»0.125«g»
«% purity = × 100 =» 70.6 «%»
ALTERNATIVE 2:
«mol of pure hydrated ethanedioic acid in 1 dm3 solution = 7.00 × 10-4 × =» 2.80×10-2 «mol»
«mass of pure hydrated ethanedioic acid in sample = 2.80 × 10-2 mol × 126.08 g mol-1 =» 3.53 «g»
«% purity = × 100 =» 70.6 «%»
ALTERNATIVE 3:
mol of hydrated ethanedioic acid (assuming sample to be pure) = = 0.03966 «mol»
actual amount of hydrated ethanedioic acid = «7.00 × 10-4 × =» 2.80 × 10-2 «mol»
«% purity = × 100 =» 70.6 «%»
Award suitable part marks for alternative methods.
Award [3] for correct final answer.
Award [2 max] for 50.4 % if anhydrous ethanedioic acid assumed.
electrons delocalized «across the O–C–O system»
OR
resonance occurs
Accept delocalized π-bond(s).
122 «pm» < C–O < 143 «pm»
Accept any answer in the range 123 «pm» to 142 «pm». Accept “bond intermediate between single and double bond” or “bond order 1.5”.
Examiners report
Trends in physical and chemical properties are useful to chemists.
The Activity series lists the metal in order of reactivity.
Explain the general increasing trend in the first ionization energies of the period 3 elements, Na to Ar.
Explain why the melting points of the group 1 metals (Li → Cs) decrease down the group.
State an equation for the reaction of phosphorus (V) oxide, P4O10 (s), with water.
Describe the emission spectrum of hydrogen.
Identify the strongest reducing agent in the given list.
A voltaic cell is made up of a Mn2+/Mn half-cell and a Ni2+/Ni half-cell.
Deduce the equation for the cell reaction.
The voltaic cell stated in part (ii) is partially shown below.
Draw and label the connections needed to show the direction of electron movement and ion flow between the two half-cells.
Markscheme
increasing number of protons
OR
increasing nuclear charge
«atomic» radius/size decreases
OR
same number of shells
OR
similar shielding «by inner electrons»
«greater energy needed to overcome increased attraction between nucleus and electrons»
atomic/ionic radius increases
smaller charge density
OR
force of attraction between metal ions and delocalised electrons decreases
Do not accept discussion of attraction between valence electrons and
nucleus for M2.
Accept “weaker metallic bonds” for M2.
P4O10 (s) + 6H2O (l) → 4H3PO4 (aq)
Accept “P4O10 (s) + 2H2O (l) → 4HPO3 (aq)” (initial reaction).
«series of» lines
OR
only certain frequencies/wavelengths
convergence at high«er» frequency/energy/short«er» wavelength
M1 and/or M2 may be shown on a diagram.
Mn
Mn (s) + Ni2+ (aq) → Ni (s) + Mn2+ (aq)
wire between electrodes AND labelled salt bridge in contact with both electrolytes
anions to right (salt bridge)
OR
cations to left (salt bridge)
OR
arrow from Mn to Ni (on wire or next to it)
Electrodes can be connected directly or through voltmeter/ammeter/light bulb, but not a battery/power supply.
Accept ions or a specific salt as the label of the salt bridge.
Examiners report
Ammonia, NH3, is industrially important for the manufacture of fertilizers, explosives and plastics.
Ammonia is produced by the Haber–Bosch process which involves the equilibrium:
N2 (g) + 3 H2 (g) 2 NH3 (g)
The effect of temperature on the position of equilibrium depends on the enthalpy change of the reaction.
Ammonia is soluble in water and forms an alkaline solution:
NH3 (g) + H2O (l) NH4+ (aq) + HO– (aq)
Draw arrows in the boxes to represent the electron configuration of a nitrogen atom.
Draw the Lewis (electron dot) structure of the ammonia molecule.
Deduce the expression for the equilibrium constant, Kc, for this equation.
Explain why an increase in pressure shifts the position of equilibrium towards the products and how this affects the value of the equilibrium constant, Kc.
State how the use of a catalyst affects the position of the equilibrium.
Determine the enthalpy change, ΔH, for the Haber–Bosch process, in kJ. Use Section 11 of the data booklet.
Calculate the enthalpy change, ΔH⦵, for the Haber–Bosch process, in kJ, using the following data.
.
Suggest why the values obtained in (d)(i) and (d)(ii) differ.
State the relationship between NH4+ and NH3 in terms of the Brønsted–Lowry theory.
Determine the concentration, in mol dm–3, of the solution formed when 900.0 dm3 of NH3 (g) at 300.0 K and 100.0 kPa, is dissolved in water to form 2.00 dm3 of solution. Use sections 1 and 2 of the data booklet.
Calculate the concentration of hydroxide ions in an ammonia solution with pH = 9.3. Use sections 1 and 2 of the data booklet.
Markscheme
Accept all 2p electrons pointing downwards.
Accept half arrows instead of full arrows.
Accept lines or dots or crosses for electrons, or a mixture of these
✔
shifts to the side with fewer moles «of gas»
OR
shifts to right as there is a reduction in volume✔
«value of » Kc unchanged ✔
Accept “Kc only affected by changes in temperature”.
same/unaffected/unchanged ✔
bonds broken: N≡N + 3(H–H) / «1 mol×»945 «kJ mol–1» + 3«mol»×436 «kJ mol–1» / 945 «kJ» + 1308 «kJ» / 2253 «kJ» ✔
bonds formed: 6(N–H) / 6«mol»×391 «kJ mol–1» / 2346 «kJ» ✔
ΔH = «2253 kJ – 2346 kJ = » –93 «kJ» ✔
Award [2 max] for (+)93 «kJ»
–92.4 «kJ» ✔
«N-H» bond enthalpy is an average «and may not be the precise value in NH3» ✔
Accept it relies on average values not specific to NH3
conjugate «acid and base» ✔
amount of ammonia ✔
concentration ✔
Award [2] for correct final answer.
[OH−] ✔
Examiners report
Most students realised that the three p-orbitals were all singly filled.
Even more candidates could draw the correct Lewis structure of ammonia, with omission of the lone pair being the most common error.
Most students could deduce the equilibrium constant expression from the equilibrium equation.
Many students realised that increasing pressure shifts an equilibrium to the side with the most moles of gas (though the "of gas" was frequently omitted!) but probably less than half realised that, even though the equilibrium position changes, the value of the equilibrium constant remains constant.
It was pleasing to see that about a third of students gaining full marks and an equal number only lost a single mark because they failed to locate the correct bond enthalpy for molecular nitrogen.
Very few students could determine the enthalpy change from enthalpy of formation data, with many being baffled by the absence of values for the elemental reactants and more than half who overcame this obstacle failed to note that 2 moles of ammonia are produced.
About half the candidates recognised the species as a conjugate acid-base pair, though some lost the mark by confusing the acid and base, even though this information was not asked for.
About 40% of candidates gained full marks for the calculation and a significant number of others gained the second mark to calculate the concentration as an ECF.
This question was very poorly answered with many candidates calculating the [H+] instead of [OH-].
Two hydrides of nitrogen are ammonia and hydrazine, N2H4. One derivative of ammonia is methanamine whose molecular structure is shown below.
Hydrazine is used to remove oxygen from water used to generate steam or hot water.
N2H4(aq) + O2(aq) → N2(g) + 2H2O(l)
The concentration of dissolved oxygen in a sample of water is 8.0 × 10−3 gdm−3.
Estimate the H−N−H bond angle in methanamine using VSEPR theory.
Ammonia reacts reversibly with water.
NH3(g) + H2O(l) NH4+(aq) + OH−(aq)
Explain the effect of adding H+(aq) ions on the position of the equilibrium.
Hydrazine reacts with water in a similar way to ammonia. Deduce an equation for the reaction of hydrazine with water.
Outline, using an ionic equation, what is observed when magnesium powder is added to a solution of ammonium chloride.
Hydrazine has been used as a rocket fuel. The propulsion reaction occurs in several stages but the overall reaction is:
N2H4(l) → N2(g) + 2H2(g)
Suggest why this fuel is suitable for use at high altitudes.
Determine the enthalpy change of reaction, ΔH, in kJ, when 1.00 mol of gaseous hydrazine decomposes to its elements. Use bond enthalpy values in section 11 of the data booklet.
N2H4(g) → N2(g) + 2H2(g)
The standard enthalpy of formation of N2H4(l) is +50.6 kJmol−1. Calculate the enthalpy of vaporization, ΔHvap, of hydrazine in kJmol−1.
N2H4(l) → N2H4(g)
(If you did not get an answer to (f), use −85 kJ but this is not the correct answer.)
Calculate, showing your working, the mass of hydrazine needed to remove all the dissolved oxygen from 1000 dm3 of the sample.
Calculate the volume, in dm3, of nitrogen formed under SATP conditions. (The volume of 1 mol of gas = 24.8 dm3 at SATP.)
Markscheme
107°
Accept 100° to < 109.5°.
Literature value = 105.8°
[1 mark]
removes/reacts with OH−
moves to the right/products «to replace OH− ions»
Accept ionic equation for M1.
[2 marks]
N2H4(aq) + H2O(l) N2H5+(aq) + OH–(aq)
Accept N2H4(aq) + 2H2O(l) N2H62+(aq) + 2OH–(aq).
Equilibrium sign must be present.
[1 mark]
bubbles
OR
gas
OR
magnesium disappears
2NH4+(aq) + Mg(s) → Mg2+(aq) + 2NH3(aq) + H2(g)
Do not accept “hydrogen” without reference to observed changes.
Accept "smell of ammonia".
Accept 2H+(aq) + Mg(s) → Mg2+(aq) + H2(g)
Equation must be ionic.
[2 mark]
no oxygen required
[1 mark]
bonds broken:
E(N–N) + 4E(N–H)
OR
158 «kJmol–1» + 4 x 391 «kJmol–1» / 1722 «kJ»
bonds formed:
E(N≡N) + 2E(H–H)
OR
945 «kJmol–1» + 2 x 436 «kJmol–1» / 1817 «kJ»
«ΔH = bonds broken – bonds formed = 1722 – 1817 =» –95 «kJ»
Award [3] for correct final answer.
Award [2 max] for +95 «kJ».
[3 marks]
OR
ΔHvap= −50.6 kJmol−1 − (−95 kJmol−1)
«ΔHvap =» +44 «kJmol−1»
Award [2] for correct final answer.
Award [1 max] for −44 «kJmol−1».
Award [2] for:
ΔHvap − = 50.6 kJmol−1 − (−85 kJmol−1) + = 34 «kJmol−1».
Award [1 max] for −34 «kJmol−1».
[2 marks]
total mass of oxygen «= 8.0 x 10–3 gdm–3 x 1000 dm3» = 8.0 «g»
n(O2) «» 0.25 «mol»
OR
n(N2H4) = n(O2)
«mass of hydrazine = 0.25 mol x 32.06 gmol–1 =» 8.0 «g»
Award [3] for correct final answer.
[3 marks]
«n(N2H4) = n(O2) » 0.25 «mol»
«volume of nitrogen = 0.25 mol x 24.8 dm3mol–1» = 6.2 «dm3»
Award [1] for correct final answer.
[1 mark]
Examiners report
Iron (II) sulfide reacts with hydrochloric acid to form hydrogen sulfide, H2S.
In aqueous solution, hydrogen sulfide acts as an acid.
Draw the Lewis (electron dot) structure of hydrogen sulfide.
Predict the shape of the hydrogen sulfide molecule.
State the formula of its conjugate base.
Saturated aqueous hydrogen sulfide has a concentration of 0.10 mol dm−3 and a pH of 4.0. Demonstrate whether it is a strong or weak acid.
Calculate the hydroxide ion concentration in saturated aqueous hydrogen sulfide.
A gaseous sample of nitrogen, contaminated only with hydrogen sulfide, was reacted with excess sodium hydroxide solution at constant temperature. The volume of the gas changed from 550 cm3 to 525 cm3.
Determine the mole percentage of hydrogen sulfide in the sample, stating one assumption you made.
Markscheme
OR
✔
Accept any combination of lines, dots or crosses to represent electrons.
bent/non-linear/angular/v-shaped✔
HS− ✔
weak AND strong acid of this concentration/[H+] = 0.1 mol dm−3 would have pH = 1
OR
weak AND [H+] = 10−4 < 0.1 «therefore only fraction of acid dissociated» ✔
10−10 «mol dm−3» ✔
Mole percentage H2S:
volume of H2S = «550 − 525 = » 25 «cm3» ✔
mol % H2S = « = » 4.5 «%» ✔
Award [2] for correct final answer of 4.5 «%»
Assumption:
«both» gases behave as ideal gases ✔
Accept “volume of gas mol of gas”.
Accept “reaction goes to completion”.
Accept “nitrogen is insoluble/does not react with NaOH/only H2S reacts with NaOH”.
Examiners report
Magnesium is a group 2 metal which exists as a number of isotopes and forms many compounds.
State the nuclear symbol notation, , for magnesium-26.
Mass spectroscopic analysis of a sample of magnesium gave the following results:
Calculate the relative atomic mass, Ar, of this sample of magnesium to two decimal places.
Magnesium burns in air to form a white compound, magnesium oxide. Formulate an equation for the reaction of magnesium oxide with water.
Describe the trend in acid-base properties of the oxides of period 3, sodium to chlorine.
In addition to magnesium oxide, magnesium forms another compound when burned in air. Suggest the formula of this compound
Describe the structure and bonding in solid magnesium oxide.
Magnesium chloride can be electrolysed.
Deduce the half-equations for the reactions at each electrode when molten magnesium chloride is electrolysed, showing the state symbols of the products. The melting points of magnesium and magnesium chloride are 922 K and 987 K respectively.
Anode (positive electrode):
Cathode (negative electrode):
Markscheme
«Ar =»
«= 24.3269 =» 24.33
Award [2] for correct final answer.
Do not accept data booklet value (24.31).
MgO(s) + H2O(l) → Mg(OH)2(s)
OR
MgO(s) + H2O(l) → Mg2+(aq) + 2OH–(aq)
Accept .
from basic to acidic
through amphoteric
Accept “alkali/alkaline” for “basic”.
Accept “oxides of Na and Mg: basic AND oxide of Al: amphoteric” for M1.
Accept “oxides of non-metals/Si to Cl acidic” for M2.
Do not accept just “become more acidic”
Mg3N2
Accept MgO2, Mg(OH)2, Mg(NOx)2, MgCO3.
«3-D/giant» regularly repeating arrangement «of ions»
OR
lattice «of ions»
Accept “giant” for M1, unless “giant covalent” stated.
electrostatic attraction between oppositely charged ions
OR
electrostatic attraction between Mg2+ and O2– ions
Do not accept “ionic” without description.
Anode (positive electrode):
2Cl– → Cl2(g) + 2e–
Cathode (negative electrode):
Mg2+ + 2e– → Mg(l)
Penalize missing/incorrect state symbols at Cl2 and Mg once only.
Award [1 max] if equations are at wrong electrodes.
Accept Mg (g).
Examiners report
Nickel catalyses the conversion of propanone to propan-2-ol.
Outline how a catalyst increases the rate of reaction.
Explain why an increase in temperature increases the rate of reaction.
Discuss, referring to intermolecular forces present, the relative volatility of propanone and propan-2-ol.
The diagram shows an unlabelled voltaic cell for the reaction
Label the diagram with the species in the equation.
Suggest a metal that could replace nickel in a new half-cell and reverse the electron flow. Use section 25 of the data booklet.
Describe the bonding in metals.
Nickel alloys are used in aircraft gas turbines. Suggest a physical property altered by the addition of another metal to nickel.
Markscheme
provides an alternative pathway/mechanism AND lower ✔
Accept description of how catalyst lowers (e.g. “reactants adsorb on surface «of catalyst»”, “reactant bonds weaken «when adsorbed»”).
more/greater proportion of molecules with ✔
greater frequency/probability/chance of collisions «between the molecules»
OR
more collision per unit of time/second ✔
hydrogen bonding/bonds «and dipole–dipole and London/dispersion forces are present in» propan-2-ol ✔
dipole–dipole «and London/dispersion are present in» propanone ✔
propan-2-ol less volatile AND hydrogen bonding/bonds stronger «than dipole–dipole »
OR
propan-2-ol less volatile AND «sum of all» intermolecular forces stronger ✔
✔
Accept OR .
electrostatic attraction ✔
between «a lattice of» metal/positive ions/cations AND «a sea of» delocalized electrons ✔
Accept “mobile/free electrons”.
Any of:
malleability/hardness
OR
«tensile» strength/ductility
OR
density
OR
thermal/electrical conductivity
OR
melting point
OR
thermal expansion ✔
Do not accept corrosion/reactivity or any chemical property.
Accept other specific physical properties.
Examiners report
A straight-forward question, however, half of the candidates only mentioned the lower activation energy and did not mention that this is through an alternative mechanism, so did not score the mark.
Half of the candidates gained the mark about the increased frequency of collision. Fewer candidates also clarified that a larger proportion of molecules have the activation energy.
Most candidates had the correct structure in their answers identifying the type of intermolecular forces in each compound and then comparing the strength of the two and reaching a conclusion. Some candidates did not know what was meant by volatile. Some candidates stated London dispersion forces in propanone instead of dipole-dipole.
60% of the candidates obtained the mark. Some candidates labelled the electrodes as ions indicating they do not understand the structure of a voltaic cell.
70% of the candidates answered correctly. The common mistake was to select a more reactive metal instead.
The mean mark on the question was 1.0 out of 2 marks. Mistakes included not mentioning the 'electrostatic attraction' and talking about 'nuclei attracting the delocalised electrons'. The weakest candidates discussed aspects of ionic and/or covalent bonding.
80% obtained the mark. Many candidates wrote more than one property, which should be discouraged. Incorrect answers included chemical properties such as reactivity.
The properties of elements can be predicted from their position in the periodic table.
Explain why Si has a smaller atomic radius than Al.
Explain the decrease in radius from Na to Na+.
State the condensed electron configurations for Cr and Cr3+.
Describe metallic bonding and how it contributes to electrical conductivity.
Deduce the Lewis (electron dot) structure and molecular geometry of sulfur dichloride, SCl2.
Suggest, giving reasons, the relative volatilities of SCl2 and H2O.
Consider the following equilibrium reaction:
2SO2 (g) + O2 (g) 2SO3 (g)
State and explain how the equilibrium would be affected by increasing the volume of the reaction container at a constant temperature.
Markscheme
nuclear charge/number of protons/Z/Zeff increases «causing a stronger pull on the outer electrons» ✓
same number of shells/«outer» energy level/shielding ✓
Na+ has one less energy level/shell
OR
Na+ has 2 energy levels/shells AND Na has 3 ✓
less shielding «in Na+ so valence electrons attracted more strongly to nucleus»
OR
effective nuclear charge/Zeff greater «in Na+ so valence electrons attracted more strongly to nucleus» ✓
Accept “more protons than electrons «in Na+»” OR “less electron-electron repulsion «in Na+»” for M2.
Cr:
[Ar] 4s13d5 ✓
Cr3+:
[Ar] 3d3 ✓
Accept “[Ar] 3d54s1”.
Accept “[Ar] 3d34s0”.
Award [1 max] for two correct full electron configurations “1s22s22p63s23p64s13d5 AND 1s22s22p63s23p63d3”.
Award [1 max] for 4s13d5 AND 3d3.
electrostatic attraction ✓
between «a lattice of» cations/positive «metal» ions AND «a sea of» delocalized electrons ✓
mobile electrons responsible for conductivity
OR
electrons move when a voltage/potential difference/electric field is applied ✓
Do not accept “nuclei” for “cations/positive ions” in M2.
Accept “mobile/free” for “delocalized” electrons in M2.
Accept “electrons move when connected to a cell/battery/power supply” OR “electrons move when connected in a circuit” for M3.
H2O forms hydrogen bonding «while SCl2 does not» ✓
SCl2 «much» stronger London/dispersion/«instantaneous» induced dipole-induced dipole forces ✓
Alternative 1:
H2O less volatile AND hydrogen bonding stronger «than dipole–dipole and dispersion forces» ✓
Alternative 2:
SCl2 less volatile AND effect of dispersion forces «could be» greater than hydrogen bonding ✓\
Ignore reference to Van der Waals.
Accept “SCl2 has «much» larger molar mass/electron density” for M2.
pressure decrease «due to larger volume» ✓
reactant side has more moles/molecules «of gas» ✓
reaction shifts left/towards reactants ✓
Award M3 only if M1 OR M2 is awarded.
Examiners report
There are many oxides of silver with the formula AgxOy. All of them decompose into their elements when heated strongly.
After heating 3.760 g of a silver oxide 3.275 g of silver remained. Determine the empirical formula of AgxOy.
Suggest why the final mass of solid obtained by heating 3.760 g of AgxOy may be greater than 3.275 g giving one design improvement for your proposed suggestion. Ignore any possible errors in the weighing procedure.
Naturally occurring silver is composed of two stable isotopes, 107Ag and 109Ag.
The relative atomic mass of silver is 107.87. Show that isotope 107Ag is more abundant.
Some oxides of period 3, such as Na2O and P4O10, react with water. A spatula measure of each oxide was added to a separate 100 cm3 flask containing distilled water and a few drops of bromothymol blue indicator.
The indicator is listed in section 22 of the data booklet.
Deduce the colour of the resulting solution and the chemical formula of the product formed after reaction with water for each oxide.
Explain the electrical conductivity of molten Na2O and P4O10.
Outline the model of electron configuration deduced from the hydrogen line emission spectrum (Bohr’s model).
Markscheme
n(Ag) = «» 0.03036 «mol»
AND
n(O) = «» 0.03031 «mol»
« / ratio of Ag to O approximately 1 : 1, so»
AgO
Accept other valid methods for M1.
Award [1 max] for correct empirical formula if method not shown.
[2 marks]
temperature too low
OR
heating time too short
OR
oxide not decomposed completely
heat sample to constant mass «for three or more trials»
Accept “not heated strongly enough”.
If M1 as per markscheme, M2 can only be awarded for constant mass technique.
Accept "soot deposition" (M1) and any suitable way to reduce it (for M2).
Accept "absorbs moisture from atmosphere" (M1) and "cool in dessicator" (M2).
Award [1 max] for reference to impurity AND design improvement.
[2 marks]
Ar closer to 107/less than 108 «so more 107Ag»
OR
Ar less than the average of (107 + 109) «so more 107Ag»
Accept calculations that gives greater than 50% 107Ag.
[1 mark]
Do not accept name for the products.
Accept “Na+ + OH–” for NaOH.
Ignore coefficients in front of formula.
[3 marks]
«molten» Na2O has mobile ions/charged particles AND conducts electricity
«molten» P4O10 does not have mobile ions/charged particles AND does not conduct electricity/is poor conductor of electricity
Do not award marks without concept of mobile charges being present.
Award [1 max] if type of bonding or electrical conductivity correctly identified in each compound.
Do not accept answers based on electrons.
Award [1 max] if reference made to solution.
[2 marks]
electrons in discrete/specific/certain/different shells/energy levels
energy levels converge/get closer together at higher energies
OR
energy levels converge with distance from the nucleus
Accept appropriate diagram for M1, M2 or both.
Do not give marks for answers that refer to the lines in the spectrum.
[2 marks]
Examiners report
Some physical properties of molecular substances result from the different types of forces between their molecules.
Explain why the hydrides of group 16 elements (H2O, H2S, H2Se and H2Te) are polar molecules.
The graph shows the boiling points of the hydrides of group 16 elements.

Explain the increase in the boiling point from H2S to H2Te.
Lewis structures show electron domains and are used to predict molecular geometry.
Deduce the electron domain geometry and the molecular geometry for the NH2− ion.
Markscheme
polar bonds «between H and group 16 element»
OR
difference in electronegativities «between H and group 16 element»
uneven distribution of charge/electron cloud
OR
non-linear/bent/V-shaped/angular shape «due to lone pairs»
OR
polar bonds/dipoles do not cancel out
M2:
Do not accept “net/overall dipole moment” without further explanation.
Accept “non-symmetrical «shape/distribution of charge»”.
[2 marks]
number of electrons increases
London/dispersion/instantaneous induced dipole-induced dipole forces increase
M1: Accept “Mr/Ar increases” or “molecules become larger in size/mass/surface area”.
[2 marks]
Electron domain geometry:
tetrahedral
Molecular geometry:
bent/V-shaped/angular
Both marks can be awarded for clear diagrams. Electron domain geometry requires a 3-D diagram showing the tetrahedral arrangement.
[2 marks]
Examiners report
Lewis (electron dot) structures are useful models.
Draw the Lewis (electron dot) structures of PF3 and PF4+ and use the VSEPR theory to deduce the molecular geometry of each species.
Predict with a reason, whether the molecule PF3 is polar or non-polar.
Markscheme
Accept any combination of dots, crosses and lines.
Ignore missing brackets and positive charge.
Penalize missing lone pairs once only.
Do not apply ECF for molecular geometry.
polar AND bond polarities/dipoles do not cancel out
OR
polar AND unsymmetrical distribution of charge
Apply ECF from part (a) molecular geometry.
Examiners report
Bromine can form the bromate(V) ion, BrO3−.
State the electron configuration of a bromine atom.
Sketch the orbital diagram of the valence shell of a bromine atom (ground state) on the energy axis provided. Use boxes to represent orbitals and arrows to represent electrons.
Draw the Lewis (electron dot) structure for BrO3− that obeys the octet rule.
Predict, using the VSEPR theory, the geometry of the BrO3− ion and the O−Br−O bond angles.
Bromate(V) ions act as oxidizing agents in acidic conditions to form bromide ions.
Deduce the half-equation for this reduction reaction.
Bromate(V) ions oxidize iron(II) ions, Fe2+, to iron(III) ions, Fe3+.
Deduce the equation for this redox reaction.
Markscheme
1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p5
OR
[Ar] 4s2 3d10 4p5 ✔
Accept 3d before 4s.
Accept double-headed arrows.
Accept dots, crosses or lines to represent electron pairs.
Geometry:
trigonal/pyramidal ✔
Reason:
three bonds AND one lone pair
OR
four electron domains ✔
O−Br−O angle:
107° ✔
Accept “charge centres” for “electron domains”.
Accept answers in the range 104–109°.
BrO3− (aq) + 6e− + 6H+ (aq) → Br− (aq) + 3H2O (l)
correct reactants and products ✔
balanced equation ✔
Accept reversible arrows.
BrO3− (aq) + 6Fe2+ (aq) + 6H+ (aq) → Br− (aq) + 3H2O (l) + 6Fe3+ (aq) ✔
Examiners report
Compound A is in equilibrium with compound B.
Predict the electron domain and molecular geometries around the oxygen atom of molecule A using VSEPR.
The IR spectrum of one of the compounds is shown:
COBLENTZ SOCIETY. Collection © 2018 copyright by the U.S. Secretary of Commerce on behalf of the United States of America. All rights reserved.
Deduce, giving a reason, the compound producing this spectrum.
Compound A and B are isomers. Draw two other structural isomers with the formula .
The equilibrium constant, , for the conversion of A to B is in water at .
Deduce, giving a reason, which compound, A or B, is present in greater concentration when equilibrium is reached.
Markscheme
Electron domain geometry: tetrahedral ✔
Molecular geometry: bent/V-shaped ✔
B AND absorption/
OR
B AND absence of ✔
Accept any value between .
Accept any two isomers except for propanone and propen-2-ol:
✔✔
Penalize missing hydrogens in displayed structural formulas once only.
AND is greater than /large ✔
Examiners report
Half of the candidates answered correctly. The rest of the candidates often answered the question in terms of the carbon atom indicating that they did not read the question carefully.
About 50% of the candidates answered correctly. Quite a few, however, gave compounds other than A or B, indicating not reading the question properly or being confused by the skeletal formulas given in the question.
Nearly half of the candidates gave two correct isomers. Propanal was often given as one of the isomers. Some candidates repeated the compounds given in the question and a few gave structures with 5 bonds on a carbon atom.
Half of the candidates answered correctly. A common mistake was K > 0 instead of K > 1.
Magnesium is a reactive metal often found in alloys.
Organomagnesium compounds can react with carbonyl compounds. One overall equation is:
Compound B can also be prepared by reacting an alkene with water.
Iodomethane is used to prepare CH3Mg. It can also be converted into methanol:
CH3 + HO– → CH3OH + –
Magnesium can be produced by the electrolysis of molten magnesium chloride.
Write the half-equation for the formation of magnesium.
Suggest an experiment that shows that magnesium is more reactive than zinc, giving the observation that would confirm this.
State the name of Compound A, applying International Union of Pure and Applied Chemistry (IUPAC) rules.
Identify the strongest force between the molecules of Compound B.
Draw the structural formula of the alkene required.
Deduce the structural formula of the repeating unit of the polymer formed from this alkene.
Deduce what would be observed when Compound B is warmed with acidified aqueous potassium dichromate (VI).
Identify the type of reaction.
Outline the requirements for a collision between reactants to yield products.
The polarity of the carbon–halogen bond, C–X, facilitates attack by HO–.
Outline, giving a reason, how the bond polarity changes going down group 17.
Markscheme
Mg2+ + 2 e- → Mg ✔
Do not penalize missing charge on electron.
Accept equation with equilibrium arrows.
Alternative 1
put Mg in Zn2+(aq) ✔
Zn/«black» layer forms «on surface of Mg» ✔
Award [1 max] for “no reaction when Zn placed in Mg2+(aq)”.
Alternative 2
place both metals in acid ✔
bubbles evolve more rapidly from Mg
OR
Mg dissolves faster ✔
Alternative 3
construct a cell with Mg and Zn electrodes ✔
bulb lights up
OR
shows (+) voltage
OR
size/mass of Mg(s) decreases «over time»
OR
size/mass of Zn increases «over time»
Accept “electrons flow from Mg to Zn”.
Accept Mg is negative electrode/anode
OR
Zn is positive electrode/cathode
Accept other correct methods.
propanone ✔
Accept 2-propanone and propan-2-one.
hydrogen bonds ✔
Do not penalize missing brackets or n.
Do not award mark if continuation bonds are not shown.
no change «in colour/appearance/solution» ✔
«nucleophilic» substitution
OR
SN2 ✔
Accept “hydrolysis”.
Accept SN1
energy/E ≥ activation energy/Ea ✔
correct orientation «of reacting particles»
OR
correct geometry «of reacting particles» ✔
decreases/less polar AND electronegativity «of the halogen» decreases ✔
Accept “decreases” AND a correct comparison of the electronegativity of two halogens.
Accept “decreases” AND “attraction for valence electrons decreases”.
Examiners report
Unfortunately, only 40% of the students could write this quite straightforward half equation.
Many candidates gained some credit by suggesting voltaic cell or a displacement reaction, but most could not gain the second mark and the reason was often a failure to be able to differentiate between "what occurs" and "what is observed".
Even though superfluous numbers (2-propanone, propan-2-one) were overlooked, only about half of the students could correctly name this simple molecule.
Probably just over half the students correctly identified hydrogen bonding, with dipole-dipole being the most common wrong answer, though a significant number identified an intramolecular bond.
Few candidates could correctly eliminate water to deduce the identity of the required reactant.
Correct answers to this were very scarce and even when candidates had an incorrect alkene for the previous part, they were unable to score an ECF mark, by deducing the formula of the polymer it would produce.
Some students deduced that, as it was a tertiary alcohol, there would be no reaction, but almost all were lucky that this was accepted as well as the correct observation - "it would remain orange".
About a quarter of the students identified this as a substitution reaction, though quite a number then lost the mark by incorrectly stating it was either "free radical" or "electrophilic". A very common wrong answer was "displacement" or "single displacement" and this makes one wonder whether this terminology is being taught instead of substitution
Generally well done with the vast majority of students correctly citing "correct orientation" and many only failed to gain the second mark through failing to equate the energy required to the activation energy.
Another question that was not well answered with probably only a quarter of candidates stating that the polarity would decrease because of decreasing electronegativity down the group.
Iron may be extracted from iron (II) sulfide, FeS.
Iron (II) sulfide, FeS, is ionically bonded.
The first step in the extraction of iron from iron (II) sulfide is to roast it in air to form iron (III) oxide and sulfur dioxide.
Outline why metals, like iron, can conduct electricity.
Justify why sulfur is classified as a non-metal by giving two of its chemical properties.
Describe the bonding in this type of solid.
State the full electron configuration of the sulfide ion.
Outline, in terms of their electronic structures, why the ionic radius of the sulfide ion is greater than that of the oxide ion.
Suggest why chemists find it convenient to classify bonding into ionic, covalent and metallic.
Write the equation for this reaction.
Deduce the change in the oxidation state of sulfur.
Suggest why this process might raise environmental concerns.
Explain why the addition of small amounts of carbon to iron makes the metal harder.
Markscheme
mobile/delocalized «sea of» electrons
Any two of:
forms acidic oxides «rather than basic oxides» ✔
forms covalent/bonds compounds «with other non-metals» ✔
forms anions «rather than cations» ✔
behaves as an oxidizing agent «rather than a reducing agent» ✔
Award [1 max] for 2 correct non-chemical properties such as non-conductor, high ionisation energy, high electronegativity, low electron affinity if no marks for chemical properties are awarded.
electrostatic attraction ✔
between oppositely charged ions/between Fe2+ and S2− ✔
1s2 2s2 2p6 3s2 3p6 ✔
Do not accept “[Ne] 3s2 3p6”.
«valence» electrons further from nucleus/extra electron shell/ electrons in third/3s/3p level «not second/2s/2p»✔
Accept 2,8 (for O2–) and 2,8,8 (for S2–)
allows them to explain the properties of different compounds/substances
OR
enables them to generalise about substances
OR
enables them to make predictions ✔
Accept other valid answers.
4FeS(s) + 7O2(g) → 2Fe2O3(s) + 4SO2(g) ✔
Accept any correct ratio.
+6
OR
−2 to +4 ✔
Accept “6/VI”.
Accept “−II, 4//IV”.
Do not accept 2− to 4+.
sulfur dioxide/SO2 causes acid rain ✔
Accept sulfur dioxide/SO2/dust causes respiratory problems
Do not accept just “causes respiratory problems” or “causes acid rain”.
disrupts the regular arrangement «of iron atoms/ions»
OR
carbon different size «to iron atoms/ions» ✔
prevents layers/atoms sliding over each other ✔
Examiners report
Properties of elements and their compounds can be related to the position of the elements in the periodic table.
Explain the decrease in atomic radius from Na to Cl.
Explain why the radius of the sodium ion, Na+, is smaller than the radius of the oxide ion, O2−.
State a physical property of sodium oxide.
Markscheme
nuclear charge/number of protons/Zeff increases «causing a stronger pull on the outer electrons» ✔
same number of shells/«outer» energy level/shielding ✔
Accept “atomic number” for “number of protons”.
isoelectronic/same electronic configuration/«both» have 2.8 ✔
more protons in Na+ ✔
Any one of:
brittle ✔
high melting point/crystalline/solid «at room temperature» ✔
low volatility ✔
conducts electricity when molten ✔
does not conduct electricity at room temperature ✔
Do not accept soluble in water.
Ignore any chemical properties.
Examiners report
Both vinegar (a dilute aqueous solution of ethanoic acid) and bleach are used as cleaning agents.
Bleach reacts with ammonia, also used as a cleaning agent, to produce the poisonous compound chloramine, NH2Cl.
Outline why ethanoic acid is classified as a weak acid.
A solution of bleach can be made by reacting chlorine gas with a sodium hydroxide solution.
Cl2 (g) + 2NaOH (aq) NaOCl (aq) + NaCl (aq) + H2O (l)
Suggest, with reference to Le Châtelier’s principle, why it is dangerous to mix vinegar and bleach together as cleaners.
Draw a Lewis (electron dot) structure of chloramine.
Deduce the molecular geometry of chloramine and estimate its H–N–H bond angle.
Molecular geometry:
H–N–H bond angle:
Markscheme
partial dissociation «in aqueous solution» [✔]
ethanoic acid/vinegar reacts with NaOH [✔]
moves equilibrium to left/reactant side [✔]
releases Cl2 (g)/chlorine gas
OR
Cl2 (g)/chlorine gas is toxic [✔]
Note: Accept “ethanoic acid produces H+ ions”.
Accept “ethanoic acid/vinegar reacts with NaOCl”.
Do not accept “2CH3COOH + NaOCl + NaCl → 2CH3COONa + Cl2 + H2O” as it does not refer to equilibrium.
Accept suitable molecular or ionic equations for M1 and M3.
[✔]
Note: Accept any combination of dots/crosses or lines to represent electron pairs.
Molecular geometry:
«trigonal» pyramidal [✔]
H–N–H bond angle:
107° [✔]
Note: Accept angles in the range of 100–109.
Examiners report
The definition of a weak acid was generally correct.
Explaining why it was dangerous to mix chlorine with vinegar was not well answered but most students gained at least one mark for stating that “chlorine gas will be produced”, but couldn’t link it to equilibrium ideas.
The Lewis structure of chloramine was correct for strong candidates, but many made the mistake of omitting electron pairs on N and Cl.
The molecular geometry and bond angles often did not correspond to each other with quite a few candidates stating trigonal planar and then 107 for the angle.
The following shows some compounds which can be made from ethene, C2H4.
ethene (C2H4) → C2H5Cl → C2H6O → C2H4O
State the type of reaction which converts ethene into C2H5Cl.
Write an equation for the reaction of C2H5Cl with aqueous sodium hydroxide to produce a C2H6O compound, showing structural formulas.
Write an equation for the complete combustion of the organic product in (b).
Determine the enthalpy of combustion of the organic product in (b), in kJ mol−1, using data from section 11 of the data booklet.
State the reagents and conditions for the conversion of the compound C2H6O, produced in (b), into C2H4O.
Explain why the compound C2H6O, produced in (b), has a higher boiling point than compound C2H4O, produced in d(i).
Ethene is often polymerized. Draw a section of the resulting polymer, showing two repeating units.
Markscheme
«electrophilic» addition ✔
NOTE: Do not accept “nucleophilic addition” or “free radical addition”.
Do not accept “halogenation”.
CH3CH2Cl (g) + OH− (aq) → CH3CH2OH (aq) + Cl− (aq)
OR
CH3CH2Cl (g) + NaOH (aq) → CH3CH2OH (aq) + NaCl (aq) ✔
C2H6O (g) + 3O2 (g) → 2CO2 (g) + 3H2O (g)
OR
CH3CH2OH (g) + 3O2 (g) → 2CO2 (g) + 3H2O (g) ✔
bonds broken:
5(C–H) + C–C + C–O + O–H + 3(O=O)
OR
5(414«kJ mol−1») + 346«kJ mol−1» + 358«kJ mol−1» + 463«kJ mol−1» + 3(498«kJ mol−1») / 4731 «kJ» ✔
bonds formed:
4(C=O) + 6(O–H)
OR
4(804«kJ mol−1») + 6(463«kJ mol−1») / 5994 «kJ» ✔
«ΔH = bonds broken − bonds formed = 4731 − 5994 =» −1263 «kJ mol−1» ✔
NOTE: Award [3] for correct final answer.
K2Cr2O7/Cr2O72−/«potassium» dichromate «(VI)» AND acidified/H+
OR
«acidified potassium» manganate(VII) / «H+» KMnO4 / «H+» MnO4− ✔
NOTE: Accept “H2SO4” or “H3PO4” for “H+”.
Do not accept “HCl”.
Accept “permanganate” for “manganate(VII)”.
distil ✔
C2H6O/ethanol: hydrogen-bonding AND C2H4O/ethanal: no hydrogen-bonding/«only» dipole–dipole forces ✔
hydrogen bonding stronger «than dipole–dipole» ✔
NOTE: Continuation bonds must be shown.
Ignore square brackets and “n”.
Examiners report
Carbonated water is produced when carbon dioxide is dissolved in water under pressure.
The following equilibria are established.
Carbon dioxide acts as a weak acid.
Soda water has sodium hydrogencarbonate, NaHCO3, dissolved in the carbonated water.
Distinguish between a weak and strong acid.
Weak acid:
Strong acid:
The hydrogencarbonate ion, produced in Equilibrium (2), can also act as an acid.
State the formula of its conjugate base.
When a bottle of carbonated water is opened, these equilibria are disturbed.
State, giving a reason, how a decrease in pressure affects the position of Equilibrium (1).
Predict, referring to Equilibrium (2), how the added sodium hydrogencarbonate affects the pH.(Assume pressure and temperature remain constant.)
100.0 cm3 of soda water contains 3.0 × 10−2 g NaHCO3.
Calculate the concentration of NaHCO3 in mol dm−3.
Identify the type of bonding in sodium hydrogencarbonate.
Between sodium and hydrogencarbonate:
Between hydrogen and oxygen in hydrogencarbonate:
Markscheme
Weak acid: partially dissociated/ionized «in solution/water»
AND
Strong acid: «assumed to be almost» completely/100 % dissociated/ionized «in solution/water» [✔]
CO32– [✔]
shifts to left/reactants AND to increase amount/number of moles/molecules of gas/CO2 (g) [✔]
«additional HCO3–» shifts position of equilibrium to left [✔]
pH increases [✔]
Note: Do not award M2 without any justification in terms of equilibrium shift in M1.
«molar mass of NaHCO3 =» 84.01 «g mol–1» [✔]
«concentration = » 3.6 × 10–3 «mol dm–3» [✔]
Note: Award [2] for correct final answer.
Between sodium and hydrogencarbonate:
ionic [✔]
Between hydrogen and oxygen in hydrogencarbonate:
«polar» covalent [✔]
Examiners report
It was rather disappointing that less than 70 % of the candidates could distinguish between weak and strong acids. Many candidates referred to pH differences.
A poorly answered question, though it discriminated very well between high-scoring and low-scoring candidates. Less than 40 % of the candidates were able to deduce the formula of the conjugate base of HCO3-. Wrong answers included water, the hydroxide ion and carbon dioxide.
This was a relatively challenging question. Only about a quarter of the candidates explained how a decrease in pressure affected the equilibrium. Some candidates stated there was no shift in the equilibrium as the number of moles is the same on both sides of the equation, not acknowledging that only gaseous substances need to be considered when deciding the direction of shift in equilibrium due to a change in pressure. Some candidates wrote that the equilibrium shifts right because the gas escapes.
This was one of the most challenging questions on the paper that required application of Le Chatelier’s Principle in an unfamiliar situation. Most candidates did not refer to equilibrium (2), as directed by the question, and hence could not gain any marks. Some candidates stated that NaHCO3 was an acid and decreased pH. Some answers had contradictions that showed poor understanding of the pH concept.
Very well answered. Most candidates calculated the molar concentration correctly.
Many candidates identified the bonding between sodium and hydrogencarbonate as ionic. A much smaller proportion of candidates identified the bonding between hydrogen and oxygen in hydrogencarbonate as covalent. The most common mistake was “hydrogen bonding”.
Carbon forms many compounds.
C60 and diamond are allotropes of carbon.
But-2-ene reacts with hydrogen bromide.
Chlorine reacts with methane.
CH4 (g) + Cl2 (g) → CH3Cl (g) + HCl (g)
Outline one difference between the bonding of carbon atoms in C60 and diamond.
State two features showing that propane and butane are members of the same homologous series.
Describe a test and the expected result to indicate the presence of carbon–carbon double bonds.
Draw the full structural formula of but-2-ene.
Write the equation for the reaction between but-2-ene and hydrogen bromide.
State the type of reaction.
Suggest two differences in the 1H NMR of but-2-ene and the organic product from (d)(ii).
Calculate the enthalpy change of the reaction, ΔH, using section 11 of the data booklet.
Draw and label an enthalpy level diagram for this reaction.
Markscheme
C60 fullerene: «each carbon is» bonded to 3 C AND diamond: bonded to 4 C
OR
C60 fullerene: delocalized/resonance AND diamond: not delocalized/no resonance
OR
C60 fullerene: single and double bonds AND diamond: single bonds ✔
Accept “C60 fullerene: sp2 AND diamond: sp3”.
Accept “C60 fullerene: trigonal planar geometry / bond angles between 109.5°/109°/108°–120° AND diamond: tetrahedral geometry / bond angle 109.5°/109°”.
Accept "bonds in fullerene are shorter/stronger/have higher bond order".
same general formula / CnH2n+2 ✔
differ by CH2/common structural unit ✔
Accept "similar chemical properties".
Accept “gradation/gradual change in physical properties”.
ALTERNATIVE 1:
Test:
add bromine «water»/Br2 (aq) ✔
Result:
«orange/brown/yellow» to colourless/decolourised ✔
Do not accept “clear” for M2.
ALTERNATIVE 2:
Test:
add «acidified» KMnO4 ✔
Result:
«purple» to colourless/decolourised/brown ✔
Accept “colour change” for M2.
ALTERNATIVE 3:
Test:
add iodine / ✔
Result:
«brown» to colourless/decolourised ✔
Accept
CH3CH=CHCH3 (g) + HBr (g) → CH3CH2CHBrCH3 (l)
OR
C4H8 (g) + HBr (g) → C4H9Br (l) ✔
«electrophilic» addition/EA ✔
Do not accept nucleophilic or free radical addition.
ALTERNATIVE 1: Any two of:
but-2-ene: 2 signals AND product: 4 signals ✔
but-2-ene: «area ratio» 3:1/6:2 AND product: «area ratio» 3:3:2:1 ✔
product: «has signal at» 3.5-4.4 ppm «and but-2-ene: does not» ✔
but-2-ene: «has signal at» 4.5-6.0 ppm «and product: does not» ✔
ALTERNATIVE 2:
but-2-ene: doublet AND quartet/multiplet/4 ✔
product: doublet AND triplet AND quintet/5/multiplet AND sextet/6/multiplet ✔
Accept “product «has signal at» 1.3–1.4 ppm «and but-2-ene: does not»”.
bond breaking: C–H + Cl–Cl / 414 «kJ mol–1» + 242 «kJ mol–1»/656 «kJ»
OR
bond breaking: 4C–H + Cl–Cl / 4 × 414 «kJ mol–1» + 242 «kJ mol–1» / 1898 «kJ» ✔
bond forming: «C–Cl + H–Cl / 324 kJ mol–1 + 431 kJ mol–1» / 755 «kJ»
OR
bond forming: «3C–H + C–Cl + H–Cl / 3 × 414 «kJ mol–1» + 324 «kJ mol–1» + 431 kJ mol–1» / 1997 «kJ» ✔
«ΔH = bond breaking – bond forming = 656 kJ – 755 kJ» = –99 «kJ» ✔
Award [3] for correct final answer.
Award [2 max] for 99 «kJ».
reactants at higher enthalpy than products ✔
ΔH/-99 «kJ» labelled on arrow from reactants to products
OR
activation energy/Ea labelled on arrow from reactant to top of energy profile ✔
Accept a double headed arrow between reactants and products labelled as ΔH for M2.
Examiners report
This was a challenging question that asked about the difference between the bonding of carbon atoms in C60 and diamond. 20% of the candidates gained the mark. The majority of the candidates did not have a specific enough answer for C60 and mentioned the pentagons and hexagons but not the number of bonds or the geometry or the bond order or the electron delocalisation. Diamond was better known to candidates as expected.
About two-thirds of the candidates scored one of the two marks and stronger candidates scored both. The most common answers were the same general formula/CnH2n+2, the difference between the compounds was CH2 and similar chemical properties. The same functional group was not accepted since alkanes do not have a functional group. Some candidates only stated that they are saturated hydrocarbons not gaining any marks.
About half of the candidates gave the bromine water test with the correct results. Iodine and KMnO4 were rarely seen in the scripts. There were candidates who used the term “clear” to mean “colourless” which was not accepted. Some candidates referred to the presence of UV light in a correct way and others in an incorrect way which was not penalized in this case. 10% of the candidates left the question blank. The most common incorrect answer was in terms of the IR absorptions. Other candidates referred to enthalpies of combustion and formation.
A well answered question. 70% of the candidates gave the correct structural formula for but-2-ene. Mistakes included too many hydrogens in the structure and an incorrect position of the C=C. Candidates should be reminded that the full structural formula requires all covalent bonds to be shown.
Half of the candidates wrote the correct equation for the reaction of but-2-ene with hydrogen bromide. Incorrect answers included hydrogen as a product. As expected, the question correlated well with highly achieving candidates.
Well answered. 60% of candidates identified the type of reaction between but-2-ene and HBr, some of them including the term “electrophilic”. ECF was generously awarded when substitution was stated based on the equation where H2 was produced in part (ii). Candidates lost the mark if they only stated “hydrobromination” without mentioning addition. Some candidates lost the mark for stating “nucleophilic” or “free radical” addition.
The comparison of the 1H NMR spectra of the two organic compounds was more challenging and 10% of the candidates left this question blank. The average mark was 0.7 out of 2 marks. Mistakes included non-specific answers that just stated “more signals” or “higher chemical shift”, and stating 3 signals in 2-bromobutane instead of 4 signals. Standard level candidates were expected to use the number of signals and the ratio of the areas under the signals to answer the question since they do not cover chemical shift, however, many of them did use the 1H NMR section in the data booklet to obtain correct answers in terms of chemical shift.
This was the best answered question on the paper. Candidates identified the bonds and used bond enthalpies to calculate the enthalpy of reaction accurately. The most common mistakes were reversing the signs of bonds broken and bonds formed, assuming two Cl-Cl bonds were broken and using an incorrect value of bond enthalpy for one of the bonds.
The majority of candidates drew the enthalpy level diagram and labelled it correctly based on their answer to part (i). Some candidates reversed the products and reactants. A few candidates did not add any labels which prevented the awarding of the second mark. With 2 marks allocated to the question the second mark was awarded for correct labeling of either ΔH or Ea.